The Umbifunnel, developed through a collaboration between Key Plastics and the Rotunda Hospital in Dublin, represents a significant advancement in cord blood collection technology. This case study examines how this innovative device addresses critical safety concerns while enhancing efficiency in obstetric and neonatal care.
Visit the Umbifunnel website: www.umbifunnel.ie
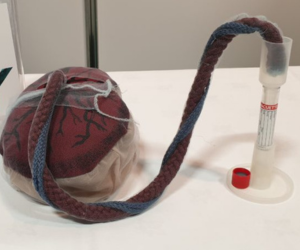
Background
Cord blood collection is an essential practice in maternity units worldwide, providing samples for various diagnostic tests including:
- Blood typing
- Genetic screening
- Haemoglobinopathy screening
- Coagulopathy screening
- Traditional collection methods involving needles and syringes or draining techniques posed significant risks and limitations
The Challenge
Prior to the Umbifunnel, healthcare providers faced several challenges:
- Risk of needlestick injuries to staff
- Potential compromise of sample integrity
- Inefficient collection processes
- Safety concerns during sample handling
The Solution
The Umbifunnel was designed as a comprehensive solution, featuring:
- A collection funnel and stand
- Needle-free design
- Compatibility with standard laboratory collection tubes
Key Features
- Safe, fast, and efficient operation
- Minimal training requirements
- Hands-free capability for cord milking
- Reduced blood clot formation
Implementation & Results
The device has been successfully implemented at the Rotunda Hospital, the world’s oldest maternity hospital. Key outcomes include:
- Eliminated needlestick injury risks
- Enhanced sample integrity
- Improved efficiency in blood collection processes
- Increased versatility in sample collection








